-
×
 Botulax 200 Unit
1 × $75.00
Botulax 200 Unit
1 × $75.00
Description
Inibio was established in September 2017. though it has been only 5 years, we have done a lot of work and still go forward.
First is the cGMP-grade production facility. it is a 7th-floor building located nearby Seoul and the international airport enables us to export. We already got kGMP.
Moreover, from the beginning, we set our facility following the global standard for USA FDA & Europe EMA. It is thanks to our experts in each department such as manufacturing, R&D, QC..
They already have experienced the whole process such as development, and FDA approval. so we got it done quickly and can make the next step go fast.
In July 2023, we received approval for the kFDA. so we are concentrating on entering the global countries
▶ cGMP grade production facilities->High quality.
▶ Free Sales Certificate approved kFDA(Ministry of Food and Drug Safety)
▶ Validation of strains by the world’s leading authority->Original strain(No strain issue).
▶ 100% purity of 900kDA protein-> Fast on set time & Zero side effect in diffusion
▶ Vacuum-drying method same as Allergan’s Product
▶ Phase 1/2, 3 clinical trial in Korea completed
▶ Completed NDA for kFDA -> Plan to launch in Korean Market end of 2023
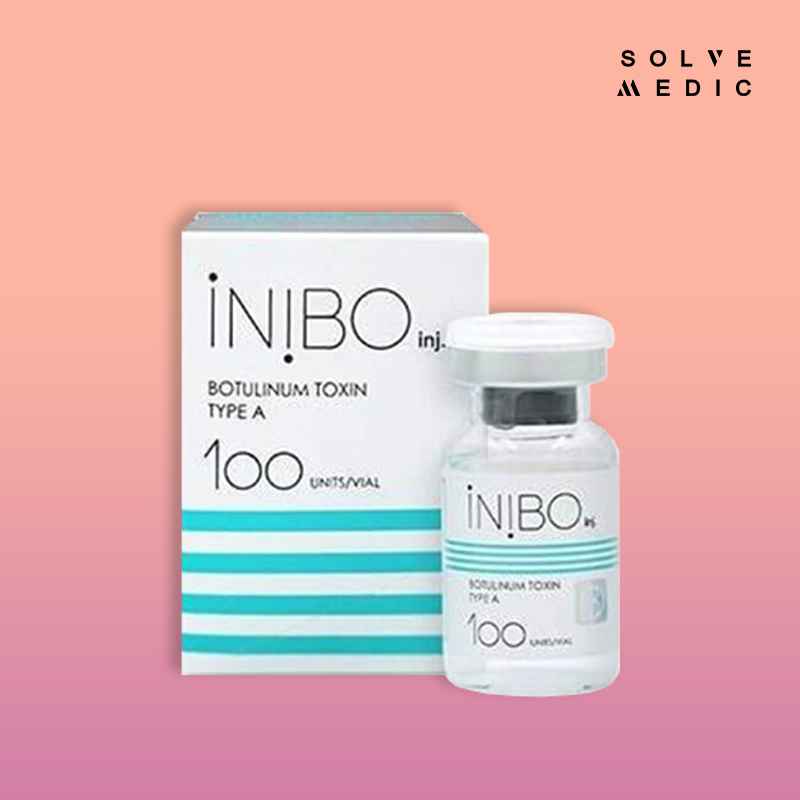
Name of Drug: INIBO inj. 100 UNITS
Pharmaceutical Dosage Form: Powder for solution for injection
Strength: 100 Units
Pack size S: 47 x 38 x 67 mm, 1 vial/box
M: 199 x 99 x 73 mm, 10 ea/box
L: 515 x 415 x 165 mm, 200 ea/box
STORAGE: Unopened vials of INIBO inj. should be stored in a refrigerator 2° to 8°C (36º to 46ºF). Do not use after the expiration date on the vial.
#INIBO #INIBO100Unit #inibobotox #iniboinj #botoxkorea #botoxforelead #botox #botoxcosmetic #aesthetics #medicalAesthetics #botilinumtoxin

















Reviews
There are no reviews yet.